- › Performance benefits › Alert management
medAspis Alert Management:
Who wants to get into trouble with the authorities?
FMD is a constant source of trouble: genuine alerts or carelessness result in suspicions of counterfeiting, which are automatically reported to the authorities.
medAspis prevents virtually all critical alerts before the pack data is even sent for verification. For the few that remain, there are clear and easy-to-understand instructions for taking action, so that your scanning staff react correctly to any alert and quarantine situations do not occur in the first place.
How do critical FMD alerts arise in the first place?
Many everyday situations lead to alerts that may indicate a suspicion of counterfeiting. This happens much more often than you think:
- Double deactivation by mistake: The error message in the case of self-inflicted double deactivation ends up directly with the authorities.
- Goods already deactivated received from supplier: Call the supplier and the goods will be returned. But the authorities will be notified, anyway.
- Unknown 2D matrix code: The code on the pill box is not recognised. Not uploaded by the manufacturer or falsification? A case for the authorities.
Why are just these cases critical?
An FMD alert message indicates that a counterfeit medicine has been found or a possible counterfeit is present. Every single suspected case must be investigated. The following two cases are critical:
- Packs with wrong 2D codes
- Already deactivated packs
What to do in these cases?
Affected packs must be separated until the problem is solved. This costs time and ties up personnel. The alerts are automatically reported to authorities and manufacturers by the NVMO. Therefore, within the scope of efficiency and quality assurance, it is crucial to avoid alerts and to react correctly to alerts when they occur.
Compliance: What rights do the authorities have?
- Investigations: Critical alert reports may lead to investigations by the competent authorities.
- Data access: The authorities have access to the FMD data of critical medicine packs for five years.
- Accountability: During this period, the authorities can investigate events and ask what happened to the packs.
medAspis Alert Management: to ensure everything runs smoothly
medAspis now has the entire FMD process perfectly under control. That’s why we can promise you not only extremely fast and efficient, but also virtually error-free processing:
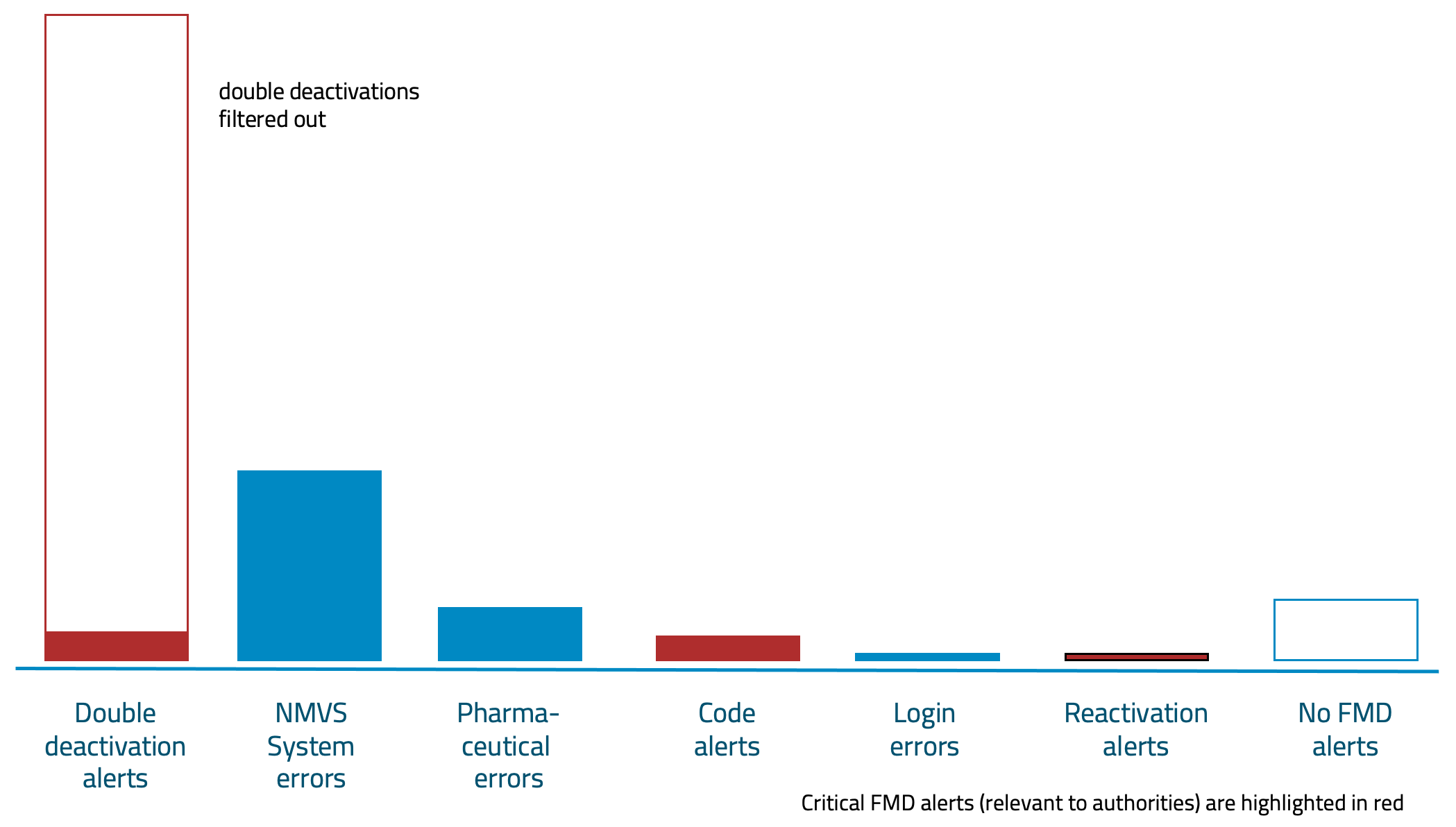
- Virtually no more alerts: The FMD Speed Scanner filters out the majority of alerts during scanning. Consequently, there are far fewer alert messages to the NMVO.
- Always react correctly:Simple and easy-to-understand instructions for scanning staff. If needs be, the medAspis Alert Team will help.
With Lean FMD, virtually no more alert messages
We have analysed all alert situations in detail and can eliminate over 80% of alert messages before they occur. Lean FMD filters out virtually every FMD alert that would otherwise be sent to the NMVS and trigger authority-relevant alerts.
For the worst case: so that everyone knows what to do
You can rely 100% on medAspis if an alert does occur. We provide all participants with exactly the information they need in their position. Clear, well structured, quality-assuring and productivity-promoting. So everybody knows for sure what to do on the spot. And the authorities are also satisfied. The FMD process cannot get any safer.
Instant scan stop in case of critical alerts. Our Alert Guard Din A2 poster shows your employees: Is the error alert? And what to do now?

medAspis provides supervisors with simple but precise explanations of all critical alerts: What are the next steps? What decision needs to be made now? What do the scanning staff need to know? All background information is easily accessible in our system.

The pharmaceutically responsible person and the quality managers receive all documents from medAspis in order to align SOPs and work instructions in the quality manual exactly according to NVMS and officially required procedures.

Good to know: The medAspis Alert Guard team is at your side for the complete and qualified processing of unresolved alerts. Just call orinform the Alert Guard Team.